Background
Obesity, a burgeoning global health crisis, has long awaited breakthrough in its management. On June 26, 2023, the New England Journal of Medicine (NEJM) published a clinical trial that might just be the turning point in this fight. The study focused on retatrutide, a novel triple hormone receptor agonist, and its efficacy on obesity treatment. This comprehensive review delves into the details of the trial, its findings, and its potential implications for the future of obesity management.
Understanding Retatrutide: A leap in Obesity Pharmacotherapy
Retatrutide represents a novel class of obesity treatment that works by simultaneously activating three different hormone receptors. Retatrutide targets glucagon-like peptide 1 (GLP-1), glucose-dependent insulinotropic polypeptide (GIP), and glucagon (GCG) receptors. In contrast, semaglutide (Wegovy®) solely targets GLP-1 and tirzepatide (Zepbound®) targets GIP in addition to GLP-1. It is postulated that, incorporating GCG receptor agonism may further enhance efficacy. This unique mechanism of action targets various aspects of obesity, including appetite regulation, energy expenditure, and glucose metabolism. The trial aimed to assess the effectiveness and safety of retatrutide in a phase 2 trial.
The Clinical Trial: Design and Execution
This phase 2 trial randomized, placebo-controlled, and featured a double-blind structure.
- Participants were adults with a body mass index (BMI) of 30 or higher or who had a BMI of 27 plus at least one weight-related condition.
- Primary end point was the efficacy of retatrutide in reducing body weight over 24 weeks.
- Secondary end points included its impact from baseline to 48 weeks and various weight reduction parameters (percent weight reduction, changes in waist circumference and BMI).
- Noteworthy exploratory end points included A1C, fasting blood glucose level, insulin and lipid levels, and blood pressure.
Results
Findings: Efficacy of Retatrutide in Weight Reduction
The results were compelling. Participants receiving retatrutide showed a significant reduction in body weight compared to placebo. The weight loss was not only statistically significant but also clinically meaningful, as it was associated with improvements in various cardiovascular health markers, including blood pressure, lipid levels, and glycemic control.
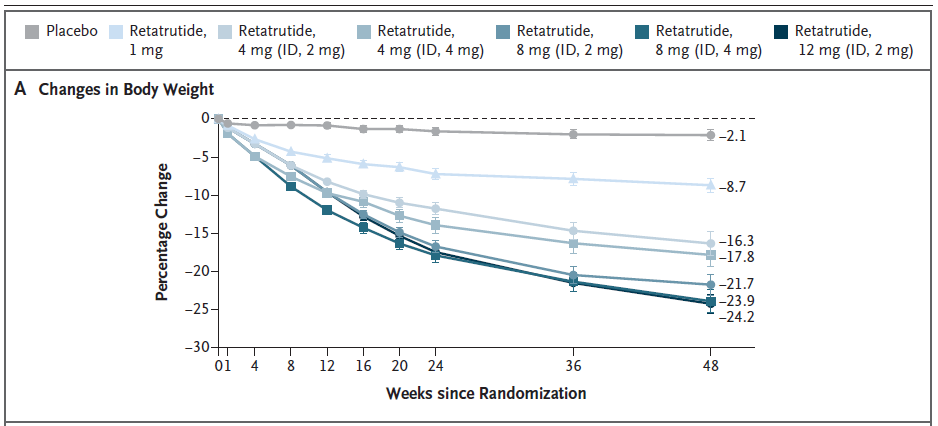
| Participant Group By Dose | Percent Change in Weight After 24 Weeks | Percent Change in Weight After 48 Weeks | Number of Participants |
|---|---|---|---|
| Placebo | -1.6% | -2.1% | 70 |
| 1 mg | -7.2% | -8.7% | 69 |
| 4 mg; ID 2 mg | -11.8% | -16.3% | 33 |
| 4 mg; ID 4 mg | -13.9% | -17.8% | 34 |
| 8 mg; ID 2 mg | -16.7% | -21.7% | 35 |
| 8 mg; ID 4 mg | -17.9% | -23.9% | 35 |
| 12 mg; ID 2 mg | -17.5% | -24.2% | 62 |
Safety Profile: Assessing the Risks
A crucial aspect of any new medication is its safety profile. The trial reported that retatrutide was well-tolerated by most participants. While some experienced mild to moderate side effects, mostly gastrointestinal related, these were generally transient and manageable. This is encouraging for the potential widespread use of retatrutide.
| Adverse Effect (AE) | Placebo | 1 mg | 4 mg; ID 2 mg | 4 mg; ID 4 mg | 8 mg; ID 2 mg | 8 mg; ID 4 mg | 12 mg; ID 2 mg |
|---|---|---|---|---|---|---|---|
| Any AE during treatment | 49 (70%) | 58 (84%) | 24 (73%) | 28 (85%) | 28 (80%) | 33 (94%) | 57 (92%) |
| Nausea | 8 (11%) | 10 (14%) | 6 (18%) | 12 (36%) | 6 (17%) | 21 (60%) | 28 (45%) |
| Vomiting | 1 (1%) | 2 (3%) | 4 (12%) | 4 (12%) | 2 (6%) | 9 (26%) | 12 (19%) |
| Diarrhea | 8 (11%) | 6 (9%) | 4 (12%) | 4 (12%) | 7 (20%) | 7 (20%) | 9 (15%) |
| Constipation | 2 (3%) | 5 (7%) | 5 (15%) | 2 (6%) | 4 (11%) | 4 (11%) | 10 (16%) |
| Antidrug ABs during treatment | 1 (1%) | 3 (4%) | 4 (12%) | 5 (16%) | 5 (16%) | 2 (6%) | 11 (18%) |
| MDD or suicidal ideation | 1 (1%) | 0 | 0 | 0 | 0 | 0 | 0 |
| Pancreatitis | 0 | 0 | 0 | 0 | 0 | 0 | 1 (2%) |
1 death (from drowning) was reported in the 4mg; ID 4 mg group. Determined to not be related to retatrutide.
Implications for Obesity Treatment and Public Health
The success of retatrutide in this trial is a significant stride in obesity treatment. Its ability to effectively reduce body weight and improve metabolic health could make it a key player in managing obesity. Numerous health issues are linked to obesity like diabetes, heart disease, and stroke. Moreover, this trial’s results could influence future research directions and healthcare policies, highlighting the importance of innovative approaches in tackling obesity and reducing further complications.
Discussion and Conclusion
Strengths/Limitations
- Strengths: extended duration phase 2 trial, approximately equal percentages of men and women, adequate sample size to extrapolate the effects of treatment on cardiovascular risk measures, and 35% of the participants identified as Hispanic or Latino
- Limitations: majority of the participants were White and the trial was limited geographically to the United States
Citation
Jastreboff AM, Kaplan LM, Frías JP, et al. Triple-Hormone-Receptor Agonist Retatrutide for Obesity – A Phase 2 Trial. N Engl J Med. 2023;389(6):514-526. doi:10.1056/NEJMoa2301972